Abstract
Background: The precise frequency of central nervous system involvement (CNS+) in patients (pts) with blastic plasmacytoid dendritic cell neoplasm (BPDCN) is currently unclear, with some studies reporting an incidence of 10% at baseline and 30% at first relapse. The CNS, a "sanctuary" for any systemic treatment, can lead to disease recurrence even after systemic response. Intrathecal (IT) chemotherapy is aimed at eradication of the disease from the CNS, with modest systemic side effects, and it is currently indicated as both prophylaxis and primary treatment. Tagraxofusp (TAG), a first-in-class CD123-targeted therapy comprising human interleukin-3 fused to a truncated diphtheria toxin payload, was approved by the US Food and Drug Administration for the treatment of pts ≥2 years of age with newly diagnosed or relapsed/refractory BPDCN in 2018, and in 2021 by the European Medicines Agency for treatment of BPDCN in first-line adult pts. Data from 5 pts with BPDCN with/without CNS+ receiving TAG and CNS treatment/prophylaxis were retrospectively collected in our center in Genoa. The aim was to evaluate if CNS treatment/prophylaxis impacts pts' prognosis and the efficacy and safety of systemic treatment with TAG.
Methods: A diagnosis of BPDCN was confirmed by histopathologic or cytologic samples with biomarkers, including CD123, CD4, and CD56, and CNS involvement was confirmed by presence of cells with the characteristic morphology and by 8-color flow cytometry for CD123. Pts received TAG intravenous infusions at 12 mcg/kg once daily on days 1-5 of a 21-day cycle. TAG was repeated for 1-4 cycles, depending on the level of response and potential bridge to hematologic stem cell transplant (HSCT). Hospitalization was mandatory for the first cycle; the following cycles were administered in an outpatient setting if no severe complications occurred during the first cycle. IT chemotherapy (methotrexate 12.5 mg, dexamethasone 4 mg, and cytarabine 50 mg) was administered at each cycle at the same doses until negative cerebrospinal fluid (CSF) was observed in CNS+ pts. After 4 treatment cycles, response was evaluated, including CSF examination.
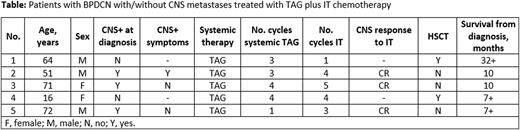
Results: TAG was administered in 5 first-line pts with BPDCN; 3 had CNS+ (1 clinically evident case and 2 with cytofluorometric positivity only). All pts received IT chemotherapy, either as a primary-intention treatment in the 3 CNS+ pts, or as prophylaxis in the 2 CNS-negative pts. The Table reports the main pt characteristics. Three of the 5 pts had CNS+ disease and achieved remission with no relapses reported at the time of the data cut for this analysis. Two pts received IT chemotherapy as CNS prophylaxis. Of these, 1 was treated with TAG followed by HSCT and continues in complete response (CR) after 32 months. The other pt received high-dose chemotherapy, achieved a partial remission after TAG, underwent HSCT, and remains alive after 7 months. Capillary leak syndrome was the most common adverse event (AE), occurring in 4/5 pts: grade (G)1, n = 1; G2, n = 2; G4, n = 1; all cases resolved. Other AEs included pulmonary aspergillosis (n = 1); sepsis (n = 1).
Conclusion: These cases illustrate that IT chemotherapy can be safely administered in pts receiving systemic TAG. Baseline disease and CNS involvement did not appear to predispose pts to different efficacy results or treatment-related AEs. IT chemotherapy effectively cleared and controlled CNS involvement. Because CNS can be a sanctuary site in BPDCN, the information from these initial cases can be helpful in the management of the disease.
Disclosures
Paley:Stemline Therapeutics: Current Employment. Riggi:Menarini/Stemline Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Angelucci:Sanofi: Speakers Bureau; Vertex: Honoraria, Other: Data monitoring committee; Roche: Consultancy; Gilead: Consultancy; Glaxo: Consultancy; Bluebird Bio: Consultancy; Celgene: Honoraria, Other: Data monitoring committee; Novartis: Honoraria; Menarini/Stemline: Consultancy; Vifopr: Honoraria, Other: Data monitoring committee.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal